🏆 Trophée des Entreprises 2025 – Clinic’nCell, à Clermont-Ferrand, simplifie et facilite les tests cliniques (Article du journal La Montagne)

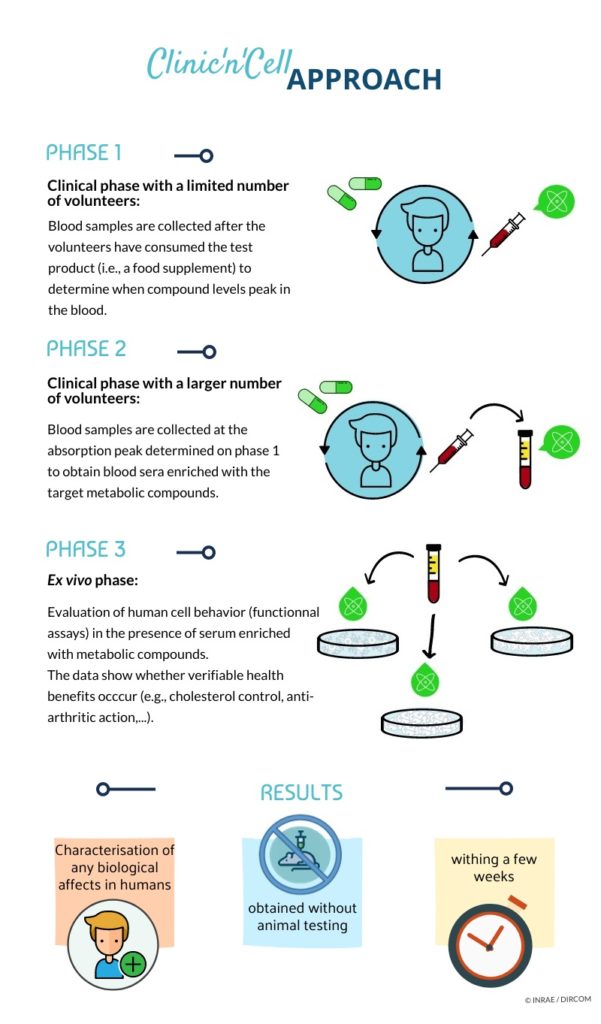
Notre méthode permet de prédire ce qui va se passer à l’intérieur du corps humain, en préalable à l’étude clinique, ce qui permet de faciliter celle-ci, de l’optimiser », explique le directeur scientifique Yohann Wittrant, l’un des cofondateurs de Clinic’n’Cell avec Fabien Wauquier, président de la société et docteur en biologie cellulaire, et Line Boutin, directrice générale (photo Franck Boileau). Clinicncell.
La méthode concerne « tout ce qui contient des principes actifs en développement », poursuit le directeur scientifique. Soit une large part de l’industrie pharmaceutique mais aussi le marché des compléments alimentaires ou encore celui de la nutricosmétique.